Merck Covid Drug
Merck is charging the US 712 for one course of its experimental Covid pill that cuts risk of hospitalization and death in half - 40 TIMES what the drug cost to make A five-day course of Mercks. The companies are looking to develop an easy-to-administer treatment for the disease.
Merck on Friday announced that its new pill to treat Covid-19 reduced the risk of hospitalization and death by about 50 percent.

Merck covid drug. October 02 2021 1331 IST Jacob Koshy October 02 2021 1328 IST. And Ridgeback Biotherapeutics dramatically reduced the number of hospitalizations from COVID-19 in a clinical trial a major finding that could mark a turning point in the coronavirus pandemic. An antiviral pill being developed by Merck Co.
Government 17 million doses of an oral antiviral treatment for COVID-19. Mercks promising experimental Covid-19 drug raises hopes for pill to fight virus. Think Tamiflu for Covid.
Merck said its new trial will study experimental drug molnupiravir for the prevention of COVID-19 among adults in the same household as someone diagnosed with symptomatic coronavirus infection. If those promising preliminary results hold the new drug could help fill a. Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus.
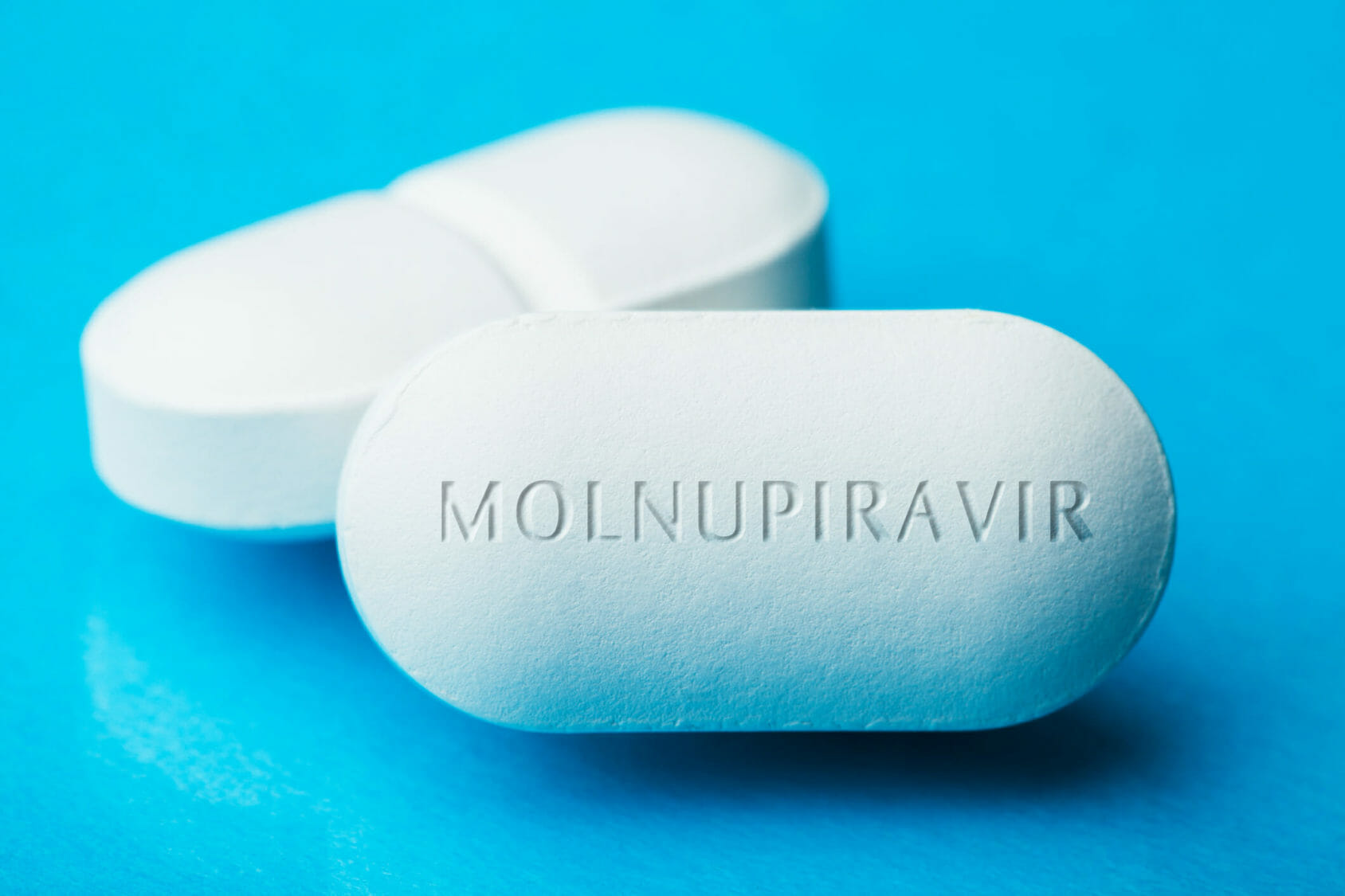
Its developers hope the pills can be prescribed widely to anyone who gets sick. Explained Molnupiravir Mercks new drug to treat COVID-19. Since molnupiravir does not target the spike protein of the virus - the target of all current COVID-19.
The drug is currently being evaluated in a. Experts laud Mercks Covid-19 drug molnupiravir. Reuters -Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus including the dominant highly transmissible Delta the company said on Wednesday.
At least three similar drugs are expected to reach the market in 2021. Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug called molnupiravir within five days of COVID. Pfizer and Merck Co announced new trials for their new experimental oral antiviral drugs for COVID-19.
Molnupiravir reduced COVID hospitalizations or death by 50 in. Merck said its new trial will study experimental drug molnupiravir for the prevention of COVID-19 among adults in the same household as someone diagnosed with symptomatic coronavirus infection. Mercks molnupiravir is among the furthest along.
Mercks drug would be the first pill to treat Covid-19. Merck plans to seek emergency authorization for the antiviral pills. Merck Co.
Pfizer said that its trial will enroll 1140 non-hospitalized adults diagnosed with the SARS-CoV-2 infection who are not at risk of severe illness. Researchers found that the medication called molnupiravir helped reduce the viral load in COVID-19 patients. At the recommendation of an independent Data Monitoring Committee and in consultation with the US.
Merck and Ridgebacks groundbreaking pill molnupiravir is leading the race of oral COVID-19 treatment. The hurdle beyond ensuring the drug. Food and Drug.
Drugmaker Merck reached an approximately 12 billion deal to supply the US. The drug named molnupiravir reduces the risk of hospitalization or death by 50 in Covid-19 patients with mild and moderate symptoms compared with placebo Merck said. Jacob Koshy October 02 2021 1328 IST Updated.
Merck says its new pill reduces deaths by half in new coronavirus patients If cleared Mercks drug would be the first pill shown to treat COVID-19 a. Said Friday that its experimental Covid-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus and that it would soon ask health officials. Merck and partner Ridgeback Biotherapeutics are already conducting a late-stage trial of the treatment in non-hospitalized patients to see if it reduces the risk of.
Pharmaceutical giant Merck announced promising results from a study of a new antiviral drug. Merck and Ridgebacks Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in. Pharmaceutical company Merck announced on March 6 2021 that its phase 2 clinical trial for an oral medication to fight COVID-19 has promising early findings.
An experimental COVID-19 treatment pill called molnupiravir being developed by Merck. Treatment with the drug called molnupiravir lowered the. Mercks COVID-19 Antiviral Drug Cuts Hospitalizations and Deaths by Half.
All you need to know The experimental drug molnupiravir significantly reduced the risk of hospitalization or death when administered to high-risk. It is likely to be followed by a number of other antiviral pills that other companies are racing to bring to market.

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say
/cloudfront-us-east-2.images.arcpublishing.com/reuters/FGX6MTMN3BLALMQRPMFRSTCQ7U.jpg)
German To Weigh Need To Purchase Merck Co S Covid 19 Drug Reuters

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg

Three Pharma Cos In Race To Find Covid 19 Treatment Pill Results Likely By 2021 End Businesstoday
Pfizer Merck Start Testing Covid Prevention Pills In Late Stage Trials Clinical Daily News Mcknight S Long Term Care News
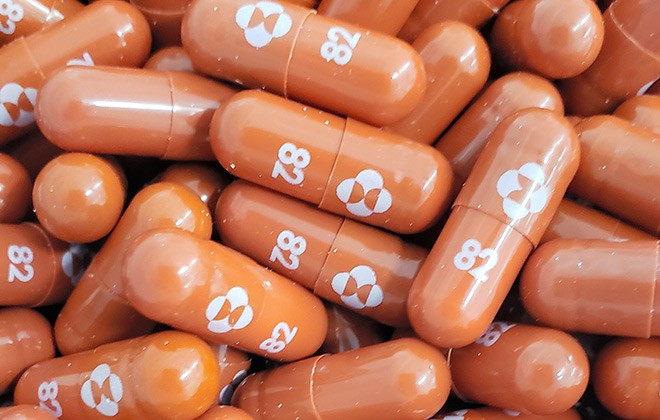
Japan Seeking Supply Of Merck S Oral Covid 19 Drug This Year The Asahi Shimbun Breaking News Japan News And Analysis







:quality(70)/d1hfln2sfez66z.cloudfront.net/10-01-2021/t_c969b405813042968e899c154998ec43_name_Merck_says_pill_to_treat_COVID19_reduces_615729f43cae215649f87100_1_Oct_01_2021_16_01_19_poster.jpg)




Komentar
Posting Komentar